| Arm | RISC-V | Renesas RL78 | Renesas RX | Renesas RH850 | STM8 | |
|
Industrial automation IEC 61508 |

IEC 61508
Pre-certified tools simplify certification for your embedded industrial automation applications. Our solutions ensure code quality for safe, fast, and on-time applications.












Typically, certifications like IEC 61508 can take up to 12 months. By using TÜV-certified development tools, you can reduce the number of steps and get your product to market faster, with less resources.
Prove standards compliance with our pre-certified embedded development tools, and use static and dynamic code analysis to continuously verify software integrity and detect vulnerabilities before deployment.
Use automated validation workflows to ensure every software update meets safety requirements, reducing risk and lowering development costs.
By using our pre-certified development tools with dedicated functional safety support services, you get access to:

| Arm | RISC-V | Renesas RL78 | Renesas RX | Renesas RH850 | STM8 | |
|
Industrial automation IEC 61508 |
Discover how the IAR platform supports IEC 61508 and other safety standards through TÜV-certified tools and pre-qualified workflows.
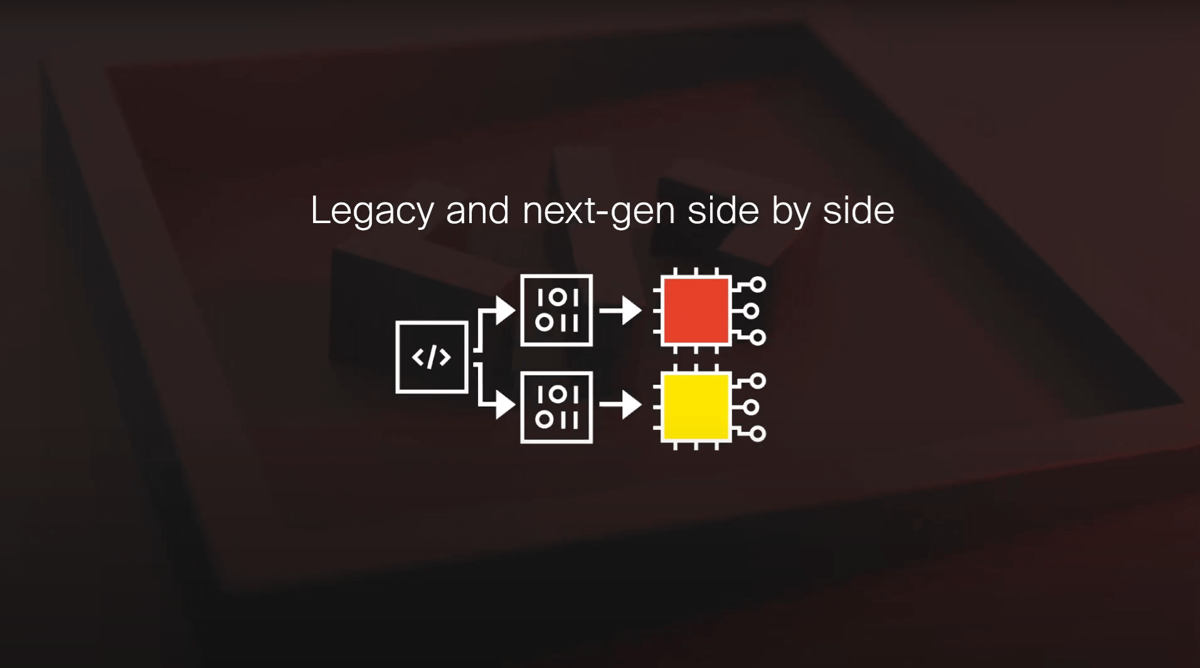
Our compilers and debuggers are fully supported in containerized environments, ensuring that every build uses the same validated environment.

Take the next step
